Contraindications: Use of a LABA, including PERFOROMIST, without an inhaled corticosteroid is contraindicated in patients with asthma. PERFOROMIST is not indicated for the treatment of asthma.
Serious Asthma-Related Events: Use of long-acting beta2-adrenergic agonists (LABA) as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone. Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
Deterioration of Disease and Acute Episodes: PERFOROMIST Inhalation Solution should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition. PERFOROMIST Inhalation Solution should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm.
Excessive Use and Use with Other LABAs: PERFOROMIST Inhalation Solution should not be used more often, at higher doses than recommended, or in conjunction with other inhaled, long-acting beta2-agonists, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
Paradoxical Bronchospasm: As with other inhaled beta2-agonists, PERFOROMIST Inhalation Solution can produce paradoxical bronchospasm that may be life threatening. If paradoxical bronchospasm occurs, PERFOROMIST Inhalation Solution should be discontinued immediately and alternative therapy instituted.
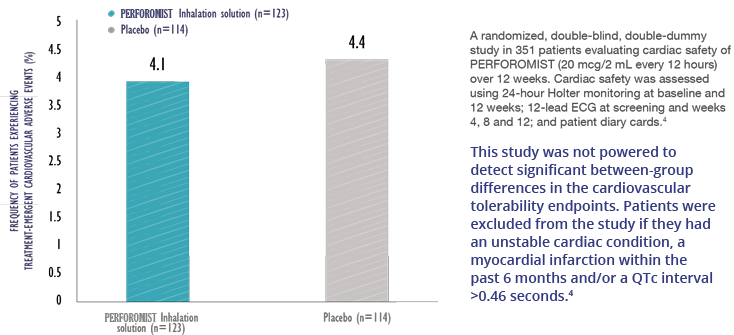
Cardiovascular Effects: PERFOROMIST Inhalation Solution, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic and/or diastolic blood pressure, and/or symptoms. PERFOROMIST Inhalation Solution should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias and hypertension.
Coexisting Conditions: PERFOROMIST Inhalation Solution, like other sympathomimetic amines, should be used with caution, especially in patients with convulsive disorders or thyrotoxicosis, and in patients who are unusually responsive to sympathomimetic amines. Doses of the related beta2-agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
Hypokalemia and Hyperglycemia: Beta-agonist medications may produce significant hypokalemia in some patients, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation. Beta-agonists can produce transient hyperglycemia.
Immediate Hypersensitivity Reactions: Immediate hypersensitivity reactions may occur after administration of PERFOROMIST Inhalation Solution, as demonstrated by cases of anaphylactic reactions, urticaria, angioedema, rash, and bronchospasm.
Use in Special Populations: PERFOROMIST Inhalation Solution, as with other beta2-agonists, should be used with extreme caution in patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval because the action of adrenergic agonists on the cardiovascular system may be potentiated by these agents.
Drug Interactions: Beta-blockers and formoterol fumarate may inhibit the effect of each other when administered concurrently. Therefore, patients with COPD should not normally be treated with beta-blockers except under certain circumstances e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-blockers in patients with COPD.
Concomitant treatment with xanthine derivatives, steroids, or diuretics may potentiate any hypokalemic effect of adrenergic agonists. The EKG changes and/or hypokalemia that may result from the administration of non-potassium sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, so caution is advised in the coadministration.
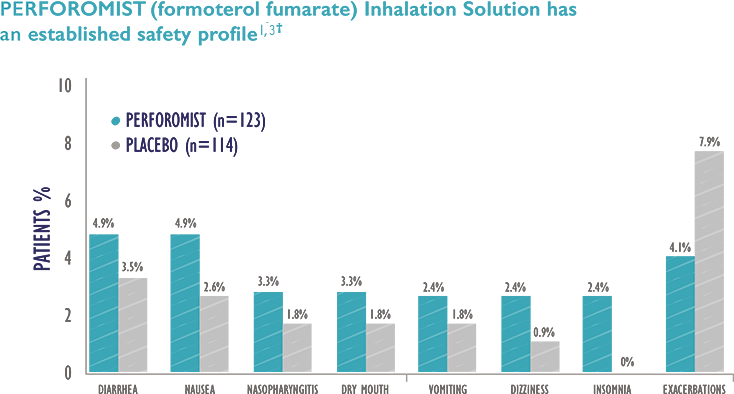
Most Common Adverse Reactions: The most common adverse reactions (≥2% and more common than placebo) with PERFOROMIST are diarrhea, nausea, nasopharyngitis, dry mouth, vomiting, dizziness, and insomnia.
Indication
PERFOROMIST® (formoterol fumarate) Inhalation Solution is indicated for the long-term, twice-daily (morning and evening) administration in the maintenance treatment of bronchoconstriction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema.
Important Limitations for Use:
- It is not indicated to treat acute deteriorations of COPD.
- It is not indicated to treat asthma. The safety and effectiveness of PERFOROMIST Inhalation Solution in asthma has not been established.
For additional information please contact us at 800-395-3376.
Click here for Full Prescribing Information.